Multiphoton Microscopy Technique 44: Two-Photon Imaging Probe for High Scattering and Deep Imaging
![]() 05/28 2024
05/28 2024
![]() 687
687
Before large-scale morphological changes occur in cells, metabolic changes first occur at the cellular level. If the metabolic state of cells can be identified, it will facilitate the diagnosis of early-stage cancer. Two-photon autofluorescence microscopy imaging can achieve cellular-level resolution and has achieved encouraging results in the detection of early cervical cancer, thus greatly promoting the development of handheld probes. The weak spontaneous emission fluorescence intensity is an important challenge impeding the clinical translation of two-photon autofluorescence imaging, and tissue scattering and imaging depth in clinical environments can further reduce fluorescence intensity. This article developed a uniquely designed imaging probe that efficiently collects two-photon excited fluorescence and images cellular metabolism under high scattering and deep imaging conditions, promising to detect early cervical cancer in clinical environments [1].
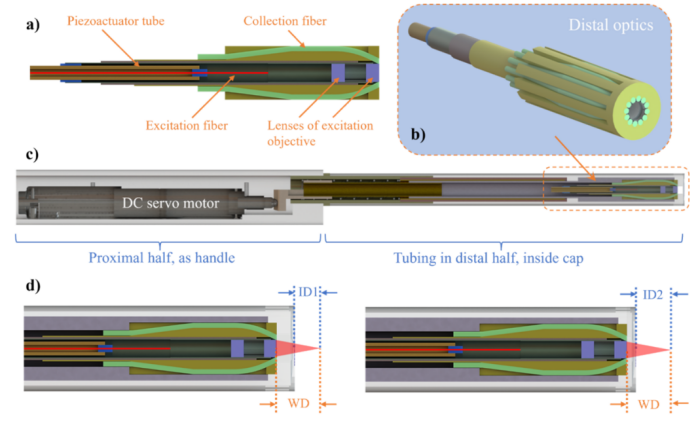
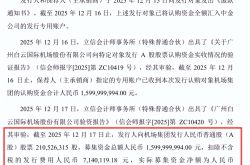
Figure 1 Optical mechanical design of the probe. a) Side sectional view of the distal optics, showing the angular bending and termination of the collection fibers. b) Three-dimensional rendered view of the distal optics, with the collection fibers and objective lens surrounded by an SLA-printed fiber holder (yellow). c) Side sectional view of the probe design. d) The working distance (WD) is constant, and the imaging depth (ID) is changed by axial drive [1].
Figure 1 shows the optical mechanical design of the probe, with the front half being an invasive component mainly used for detection and the rear half being handheld. As shown in Figure 1(a), the front half of the probe consists of a piezoelectric actuator tube, excitation fibers, an objective lens group, and collection fibers. Among them, 12 multimode fibers (NA=0.5) are radially arranged around the objective lens at a certain angle to collect spontaneously emitted fluorescence, and the excitation path and collection path of the probe are completely independent.
Figure 1(d) shows how to change the imaging distance (ID) to image tissues at different depths. First, the distance from the laser focus to the sample through the objective lens group (working distance, WD) is fixed. WD refers to the distance from the objective lens surface to the focus, and ID is the distance from the device housing surface to the focus. As shown in Figure 1(c), the rear half of the device is equipped with a DC servo motor that can achieve 6 mm axial drive and sub-micron motion resolution. When the motor is driven, the axial driving force generated by the motor is transmitted to the front optical elements through springs and balls in the device, changing the imaging distance ID and thus imaging epithelial tissues at different depths. Under conditions of an imaging depth of 50 um and a fiber angle of 25°, the authors compared the collection efficiency of the probe design adopted in this article with that of the double-cladding fiber (DCF) collection scheme.
The DCF scheme refers to using the core to transmit excitation pulses and the inner cladding to collect spontaneous fluorescence. Using DCF to collect spontaneous fluorescence is a common design scheme that allows the collection element to be axially aligned with the focus, but the size of commercial double-cladding fibers limits the overall achievable collection area, resulting in limited collection efficiency and imaging depth. Observing the collection path of DCF, although the excitation objective lens can collect a considerable proportion of emitted photons (31.8% at the front end), only 1.39% of the photons can reach the secondary cladding of DCF. Although the collection efficiency of each collection fiber of the probe in this article is only 0.52%, the combination of all 12 fibers located around the objective lens adds up to 6.24%. The 12 fibers are coupled with the photocathode of the PMT, thereby transferring the collected fluorescence to the PMT. Figure 2 shows the assembled probe installed on a three-axis translation stage. The tunable wavelength of the titanium sapphire laser is set to 775 nm, with a pulse duration of 100 fs and a repetition frequency of 80 MHz, used as the excitation source in the experiment.
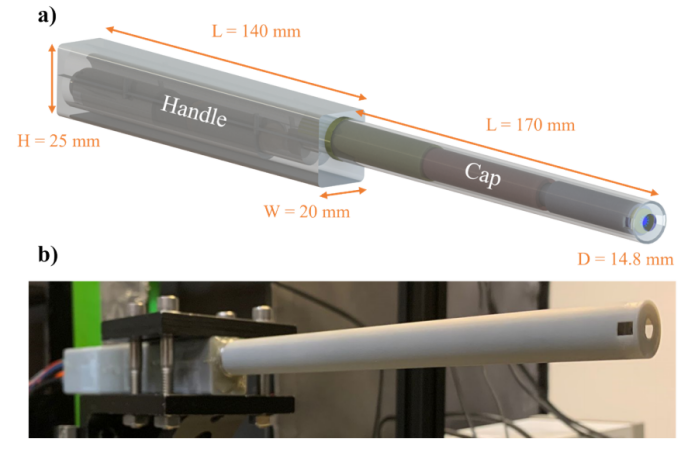
Figure 2 Optical mechanical design of the probe [1]
The authors used the probe to generate fluorescence produced by two-photon excitation in fluorescein solutions of different concentrations and recorded the voltage levels acquired by the readout module consisting of PMT, preamplifier, and data acquisition card, comparing them with a desktop microscope. The slope of the linear fit in Figure 3(a) indicates that the overall collection efficiency of the probe system reaches 55% of the system collection efficiency provided by the microscope. Figure 3(b) summarizes the analysis results of different parts of the system. Figure 3(c) shows the normalized fluorescence decay results at different imaging depths. The orange line in Figure 3(d) represents the change in fluorescence intensity with FOV radius, and the blue line shows the radial decrease in excitation power due to objective lens vignetting measured at different radial positions. The intensity decrease is closely related to the power square curve, indicating a low radial dependence of collection efficiency.
Figure 3 Characterization of the probe's collection efficiency [1]
Figure 4 shows the imaging results of the probe on pollen grain clusters, with an FOV of 120 μm for all images and an average of 10 frames. This set of images confirms that the probe proposed in this article has the ability to resolve fine features and the stability of axial scanning operations.
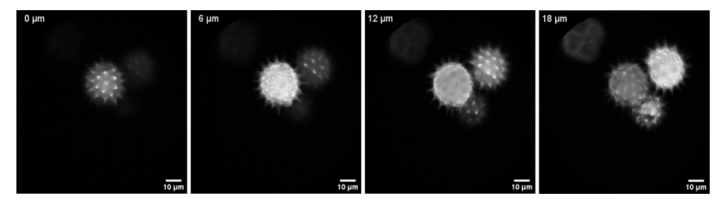
Figure 4 Two-photon images of pollen grain clusters at different axial depths obtained using the probe with 15 mW average laser power illuminating the sample surface and performing axial scanning [1]. The mucosal epithelium of porcine vocal cord tissue is very similar to that of cervical tissue, so the authors used the probe to image different depths of porcine vocal cord tissue. As shown in Figure 5, the epithelium is composed of squamous cells with bright cytoplasm and dark nuclei, and in the superficial lamina propria below an imaging depth of approximately 50 μm, the fibrous collagen layer gives way to the epithelium.
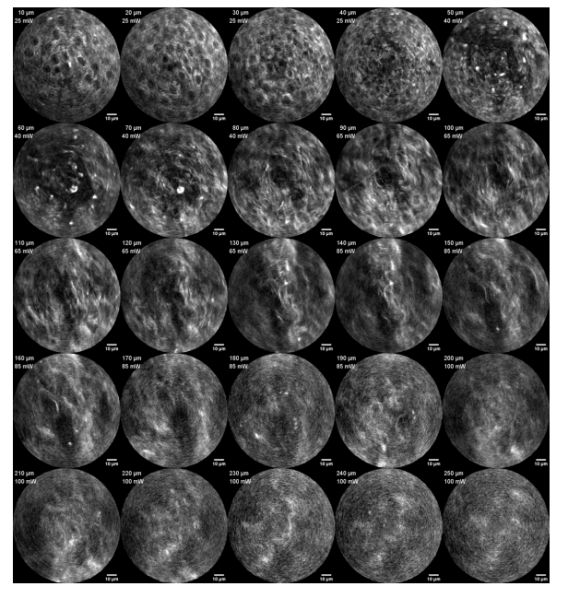
Figure 5 Spontaneous fluorescence images obtained by the probe at different imaging depths in excised porcine vocal cord tissue samples [1]
In summary, this article proposes a probe design suitable for autofluorescence collection that can achieve high collection efficiency under deep imaging and high scattering conditions. If this probe can be applied clinically, it has the potential to achieve significant breakthroughs in early cancer detection.
References:
[1] Berk Camli, Liam Andrus, Aditya Roy, Biswajit Mishra, Chris Xu, Irene Georgakoudi, Tomasz Tkaczyk, and Adela Ben-Yakar, "Two photon imaging probe with highly efficient autofluorescence collection at high scattering and deep imaging conditions," Biomed. Opt. Express 15, 3163-3182 (2024)