Quantitative Assessment of the Biomechanical Characteristics of the Crystalline Lens via Multimodal Optical Elastography Technology
![]() 09/22 2025
09/22 2025
![]() 718
718
The biomechanical attributes of the lens are intimately linked to its physiological functions and the onset and progression of various ophthalmic conditions. Nevertheless, given the lens's internal position within the eyeball and its transparent nature, attaining three-dimensional quantitative and non-destructive measurement of its Young's modulus has posed a considerable challenge in the realm of biomedical optics. This research is the first to propose integrating the quantitative capabilities of Optical Coherence Elastography (OCE) with the depth-resolving abilities of Brillouin microscopy. This integration aims to achieve a comprehensive, quantitative, and micron-resolution three-dimensional biomechanical mapping of transparent lenses, thereby paving a novel path for in vivo investigations into the biomechanical properties of lenses[1].
OCE technology facilitates direct and swift quantitative measurement of tissue Young's modulus. However, it relies on backscattered signals grounded in the principles of Optical Coherence Tomography (OCT). As a highly transparent tissue, the lens exhibits exceedingly weak internal scattering, rendering it difficult for OCE to capture signals from its deeper regions. Consequently, measurements are typically confined to the surface. Existing measurement techniques fall short of accurately mapping the three-dimensional Young's modulus distribution from the cortex to the nucleus of the lens under non-destructive conditions. This limitation hinders a profound understanding of the alterations in mechanical properties during the progression of diseases such as presbyopia and cataracts. In contrast, Brillouin microscopy harnesses spontaneous Brillouin scattering to conduct micron-resolution three-dimensional imaging of transparent samples, thereby acquiring their longitudinal modulus, also recognized as the Brillouin modulus. Nevertheless, the conversion relationship between the Brillouin modulus and the widely utilized Young's modulus in clinical practice is intricate and contingent upon the specific properties of the sample, rendering direct quantitative measurement unattainable.
This study is pioneering in proposing the amalgamation of the quantitative capabilities of OCE with the depth-resolving capabilities of Brillouin microscopy. OCE serves as a benchmark to calibrate Brillouin measurements, enabling sample-specific personalized calibration and subsequently quantifying the global measurement data from Brillouin microscopy into Young's modulus.
The Brillouin microscope employed in this study is depicted in Figure 1. It primarily comprises a laser source, a VIPA spectrometer, a stage, and associated optical pathways. The microscope operates at a wavelength of 660 nm, with an optical power of approximately 24 mW on the sample, a lateral resolution of approximately 6 μm, and an axial resolution of around 70 μm. The microscope scans along the lens axis over a range of approximately 7 mm with a step size of 10 μm and laterally over a range of about 50 μm with a step size of 2 μm. Immediately following Brillouin microscopy measurements, OCE signals from the same sample are measured.
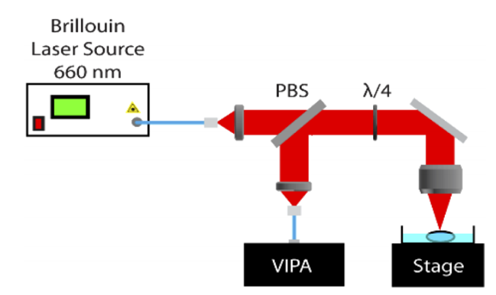
Figure 1 Schematic diagram of the Brillouin microscope utilized in this study[1]
OCE encompasses an OCT system and an air pulse generator, as illustrated in Figure 2. This study adopts a 1310 nm phase-stable swept-source OCT system, utilizing focused micro-air pulses to excite surface elastic waves. High-speed OCT scanning captures wave propagation data, and a cross-correlation algorithm computes wave velocity. Subsequently, Young's modulus is derived utilizing the surface wave equation.
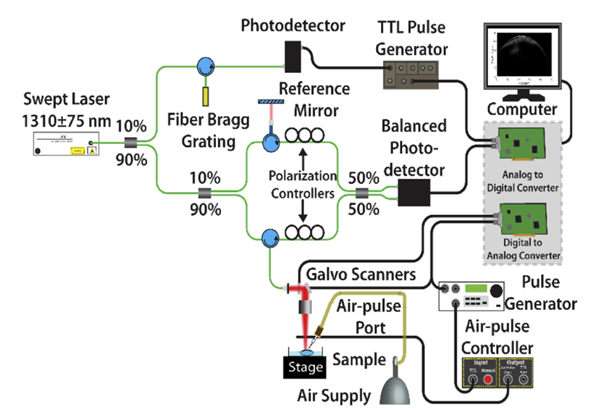
Figure 2 Schematic diagram of the OCE employed in this study[1]
This study initially utilized two systems to measure the Young's modulus and Brillouin modulus of gelatin models with varying concentrations, thereby establishing a Young's modulus-Brillouin modulus conversion relationship applicable to gelatin models and accomplishing the validation of fundamental principles. Gelatin phantoms (N = 9) with different concentrations (6%, 10%, 12%) were prepared, and their Young's modulus was verified through uniaxial mechanical testing (the gold standard). Subsequently, Young's modulus and Brillouin modulus were measured using OCE and Brillouin microscopy, respectively.
On the gelatin models, the Young's modulus measured by OCE exhibited no statistically significant difference from the mechanical testing results (p = 0.055), thereby substantiating the accuracy of OCE measurements, as demonstrated in Figure 3(a). Young's modulus and Brillouin modulus displayed a high degree of correlation (R = 0.98), with measurement results across different concentration gelatin models presented in Figure 3(b). Figure 3(c) elucidates the corresponding relationship between Brillouin modulus and Young's modulus in gelatin models. Generally, the Brillouin modulus M and Young's modulus E adhere to the empirical formula: log(M) = a log(E) + b. The measurement results in this study revealed a = 0.07 and b = 9.05 for gelatin models.
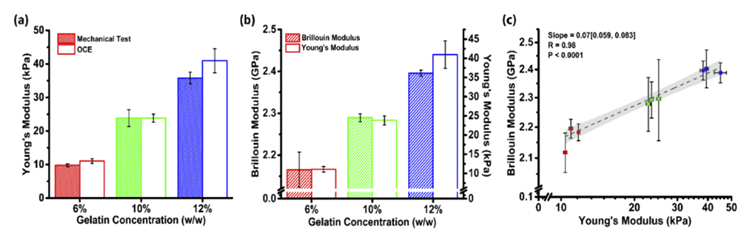
Figure 3 (a) Young's modulus measured by OCE and mechanical methods in gelatin models with different concentrations (b) Measurement results of Young's modulus and Brillouin modulus (c) Corresponding relationship between Brillouin modulus and Young's modulus[1]
Subsequently, ex vivo porcine lenses (N = 6) were employed as models to measure Young's modulus and Brillouin modulus separately. Taking into account the non-uniform characteristics of the lens, this study refrained from simply correlating the OCE surface measurements with the Brillouin average over the entire depth. Instead, it ascertained the penetration depth (approximately one wavelength) by calculating the power-weighted average frequency of the elastic wave and averaged the Brillouin modulus solely within this depth range prior to correlating it with the Young's modulus measured by OCE. This approach significantly enhanced the physical accuracy and reliability of the calibration. The measurement results for ex vivo porcine lenses in this study are presented in Figure 4(a). Fitting in accordance with the empirical formula log(M) = a log(E) + b yielded a = 0.13 and b = 9.13 for ex vivo porcine lenses.
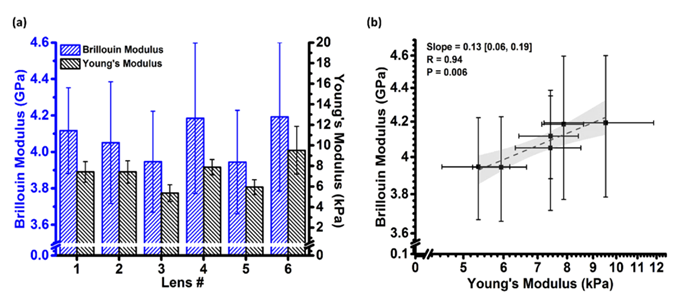
Figure 4 (a) Measurement results of Young's modulus and Brillouin modulus in ex vivo porcine lenses (b) Corresponding relationship between Brillouin modulus and Young's modulus[1]
Following calibration with OCE parameters, the three-dimensional Brillouin modulus distribution [Figure 5(a)] was globally converted into Young's modulus distribution. The variation of Young's modulus with axial depth is depicted in Figure 5(b). Notable differences in Young's modulus were observed at different depths of the porcine lens, with the Young's modulus of the lens nucleus being substantially higher than that of the anterior and posterior surfaces [Figure 5(c)]. The Young's modulus in the central region (4 - 5 mm) was 11.90 ± 2.94 kPa; the anterior (0 - 1 mm) and posterior (7 - 8 mm) regions exhibited Young's moduli of 1.98 ± 0.74 kPa and 2.93 ± 1.13 kPa, respectively. Statistical tests confirmed highly significant differences in stiffness between the nucleus and the anterior and posterior regions (p = 0.005), while no significant difference was observed between the anterior and posterior regions (p = 0.230).
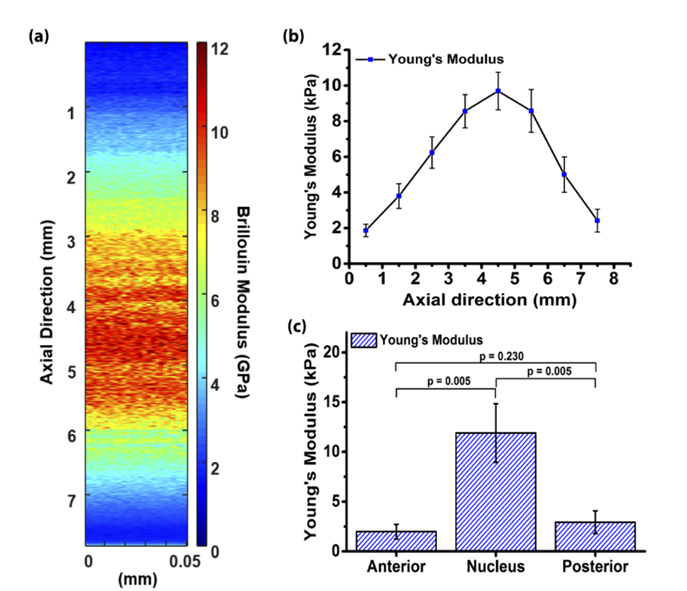
Figure 5 (a) Three-dimensional map of Brillouin modulus in ex vivo porcine lenses (b) Axial variation of Young's modulus with depth (c) Average Young's modulus of the anterior, posterior, and lens nucleus[1]
This study has successfully validated a multimodal optical elastography framework by integrating OCE with Brillouin microscopy, thereby accomplishing, for the first time, a full-depth, quantitative, and micron-resolution three-dimensional biomechanical mapping of transparent lenses. This opens up a new avenue for in vivo research on the impact of age and related diseases on the biomechanical properties of lenses.
References:
[1] Yogeshwari S. Ambekar, Manmohan Singh, Jitao Zhang, Achuth Nair, Salavat R. Aglyamov, Giuliano Scarcelli, and Kirill V. Larin, Biomed. Opt. Express 11, 2041 - 2051 (2020)