Advances in Multiphoton Microscopy: Deep Penetration and High-Resolution Photothermal Imaging
![]() 01/02 2025
01/02 2025
![]() 784
784
Label-free and non-destructive molecular imaging stands out in the biomedical field by avoiding the impact of labels on molecular properties. Currently, stimulated Raman scattering (SRS) microscopy, coherent anti-Stokes Raman scattering (CARS) microscopy, and photoacoustic (PA) microscopy are employed in various pathological studies. However, the imaging depth of SRS and CARS microscopes is constrained to a few hundred micrometers due to water absorption and tissue scattering. To enhance imaging depth, researchers have introduced PA microscopes, though these fail to achieve high spatial resolution simultaneously. This article presents a novel approach utilizing the photothermal (PT) effect induced by shortwave infrared (SWIR) light to achieve imaging that meets both millimeter-scale imaging depth and micrometer-scale resolution, outperforming existing methods [1].
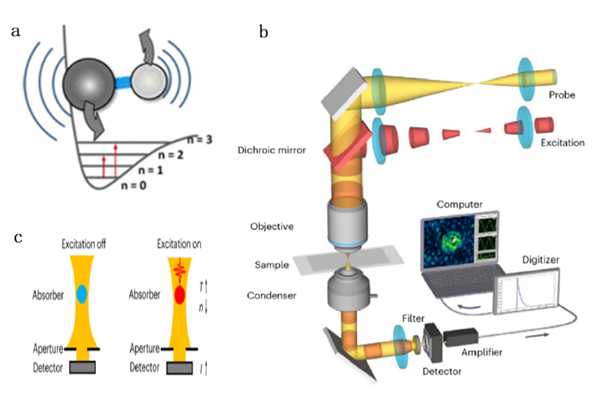
Figure 1. Principle and setup of photothermal microscopy detection [1]
The principle of photothermal imaging is illustrated in Figure 1(a). When a molecule absorbs a single photon, it generates a thermal effect, altering the local refractive index. Figure 1(b) depicts the setup, where a pulsed laser with a central wavelength of 1725 nm (corresponding to the first-order harmonic absorption peak of the C-H bond) serves as the excitation light, and a continuous laser with a central wavelength of 1310 nm acts as the probe light. Both beams are focused into the sample through a dichroic mirror to induce the photothermal effect. Figure 1(c) explains the detection principle: The excitation light interacts with the sample, causing a change in the refractive index at the absorption point, and the resulting change in probe light propagation is captured as the observation signal.
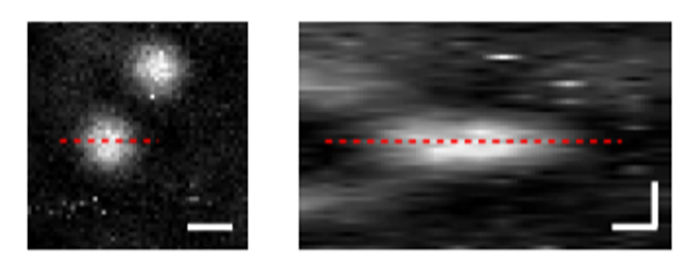
Figure 2. Imaging results of polystyrene beads using photothermal microscopy [1]
The resolution of the PT microscope was evaluated by imaging polystyrene beads with a diameter of 500 nm using the aforementioned setup, as shown in Figure 2(a). Imaging of the xy and yz interfaces and calculation of the signal-to-noise ratio revealed a lateral resolution of 0.77 µm and an axial resolution of 3.5 µm, confirming the microscope's micrometer-scale resolution.
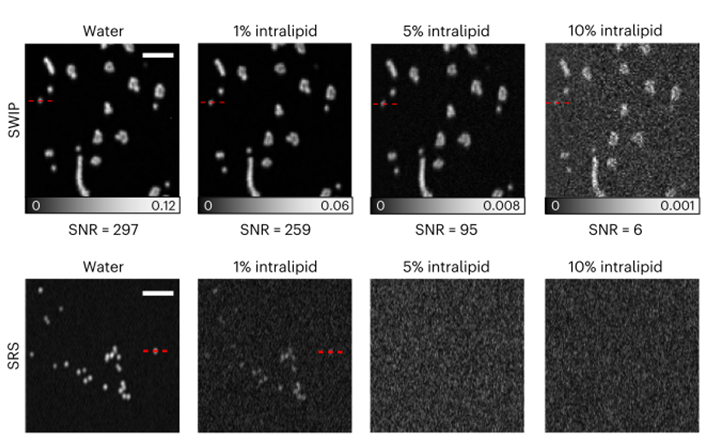
Figure 3. Comparison of imaging between photothermal microscopy (top) and stimulated Raman microscopy (bottom) [1]
Polystyrene beads were immersed in aqueous solutions of endotoxin at varying concentrations, simulating the absorption and scattering properties of biological tissues. Imaging of these solutions using PT and SRS microscopes is shown in Figure 3. As the solution concentration increases, so do the scattering and absorption effects. Notably, the signal-to-noise ratio of PT microscopy degrades more gradually under stronger scattering and absorption. At 5% and 10% concentrations, photothermal microscopy yields significantly better imaging results than SRS microscopy, achieving a penetration depth of up to 1 mm in real biological tissues.
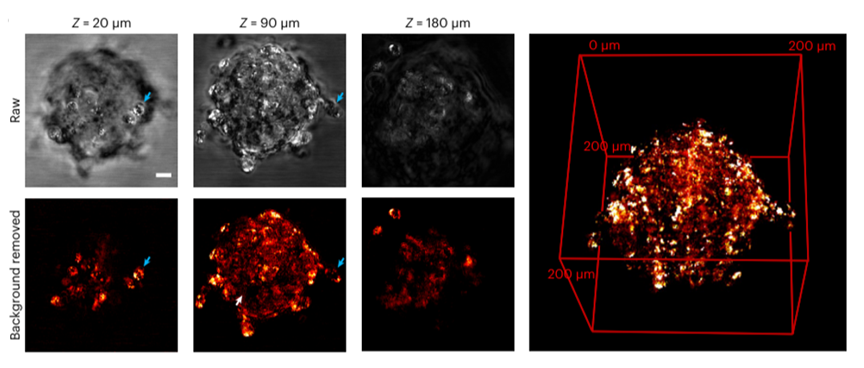
Figure 4. Three-dimensional imaging of lipids in real cells, without (top) and with (bottom) water background removal [1]
Cancer induction is closely linked to changes in cellular lipid composition, underscoring the importance of lipid imaging. The top layer in Figure 4 displays the raw image without water background signal removal, while the bottom layer shows the lipid distribution map after noise reduction using the different thermal attenuation coefficients of lipids and water. By adjusting the penetration depth, the three-dimensional lipid distribution throughout the cell was revealed, crucial for studying cancer-related pathological processes. This work introduces a novel photothermal-based imaging method that achieves both 1-millimeter penetration depth and sub-micrometer resolution, outperforming existing SRS microscopes in real biological tissues. References: [1] Ni, H., Yuan, Y., Li, M. et al. Millimetre-deep micrometre-resolution vibrational imaging by shortwave infrared photothermal microscopy. Nat. Photon. 18, 944–951 (2024).