Medical Optical Innovation: Deep Dynamic Metabolic and Structural Imaging in Living Tissues
![]() 03/24 2025
03/24 2025
![]() 962
962
From fundamental research to clinical pathology, capturing the metabolic dynamics of intact living biological systems is paramount in biomedicine. NAD(P)H and FAD, intrinsic fluorophores in biological tissues, facilitate label-free, high-resolution, and three-dimensional visualization of cellular metabolic activities through two-photon spontaneous fluorescence imaging. However, current NAD(P)H imaging depths are limited to 300 µm due to scattering and out-of-focus signals. In this study, researchers demonstrated that by leveraging a multimode fiber (MMF) source for three-photon (3P) excitation of NAD(P)H at 1100 nm, this depth limitation can be significantly overcome [1].
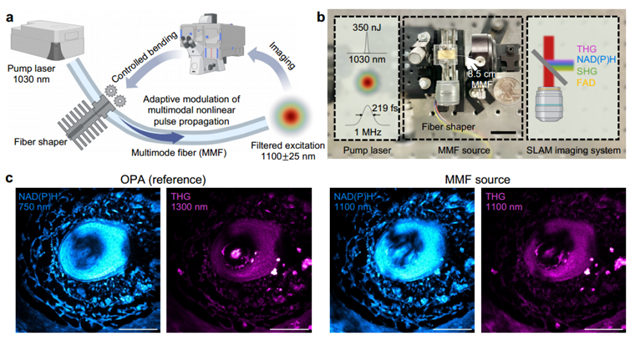
Figure 1. High-quality label-free imaging excitation source [1]
Figure 1a illustrates the excitation source schematic: ultrashort pulses with a 1 MHz repetition rate and 1030 nm wavelength traverse an 8.5 cm step-index MMF (core diameter 25 µm, 0.1 NA). A fiber shaper modulates pulse propagation to enhance beam quality and reduce pulse width. Spectral filtering at 1100 nm yields a light source ideal for NAD(P)H imaging. Figure 1c compares images from the MMF light source and an advanced optical parametric amplifier (OPA) imaging the same mouse vibrissa pad tissue region. Both methods yield comparable signal-to-noise ratios (SNR) and resolutions.
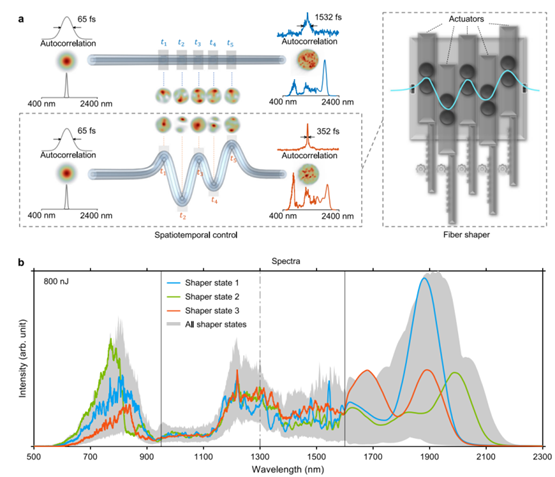
Figure 2. Spatiotemporal control of nonlinear pulse propagation in MMF via fiber shaper [2]
The fiber shaper's principle has been validated: actuator position changes apply controlled macroscopic bends to the fiber, altering nonlinear multimodal pulse propagation, thereby customizing MMF output in spectral, temporal, and spatial characteristics (Figure 2). This study integrates third-harmonic generation imaging feedback, enabling real-time actuator position adjustment for optimal spectral-temporal-spatial profiles in 3P imaging (Figure 3). Post-adjustment, 1100 nm band energy increases by 1.27 times, pulse duration shortens by 1.43 times, beam spatial profile improves, and imaging quality significantly enhances.
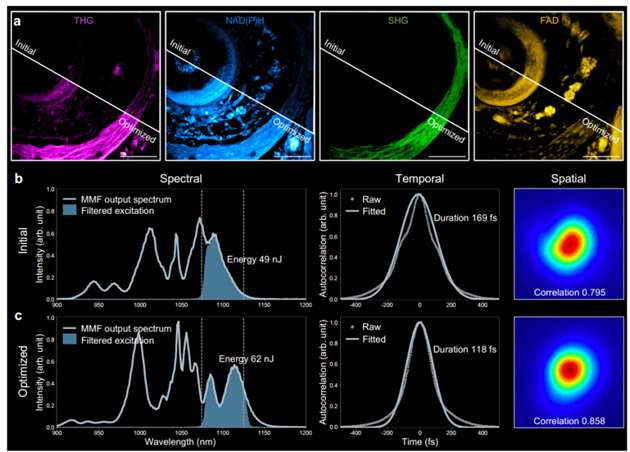
Figure 3. MMF adjustment for optimized imaging SNR and resolution [1]
To investigate NAD(P)H imaging depth limits and the effectiveness of a 1100 nm light source in extending these limits, researchers conducted spontaneous fluorescence imaging of NAD(P)H with 2P and 3P excitation using a 750 nm OPA and a 1100 nm MMF light source, respectively, on the same three-dimensional microvasculature network region. As shown in Figure 4, at superficial layers (Z ≤ 200 µm), SNR and resolution of NAD(P)H images from two-photon and three-photon fluorescence at 1100 nm are comparable. However, as imaging depth increases, two-photon fluorescence SNR declines faster due to out-of-focus background signals. Figures 4d and 4e quantify image degradation and signal attenuation in 2P and 3P nonlinear processes as a function of imaging depth.
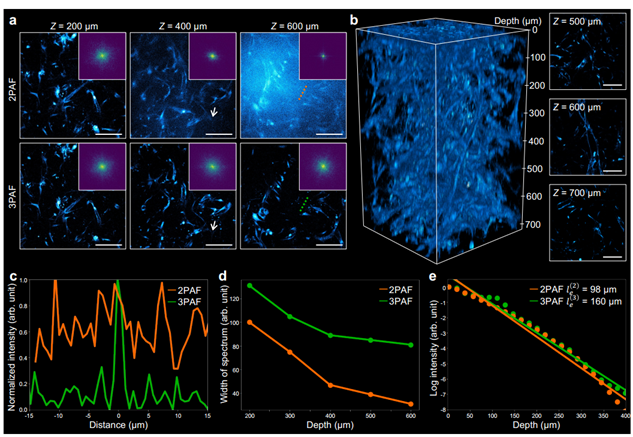
Figure 4. Deep NAD(P)H imaging achieved using a 1100 nm excitation source [1]
Prior research demonstrated that an ultrafast 1100 nm excitation source can simultaneously excite and distinguish four modalities in a multiphoton microscope, termed SLAM (simultaneous label-free autofluorescence-multiharmonic) microscopy. This study employs an MMF excitation source to drive dSLAM (dynamic SLAM) microscopy, examining its capability for non-invasive, deep metabolic and structural characterization of living multicellular systems at the subcellular level. Figure 5 displays a 350 µm×350 µm×500 µm image stack of a living blood-brain barrier microfluidic model using an MMF excitation source with a central wavelength of 1100 nm and peak power exceeding 0.5 MW. Figures 5c-i demonstrate dSLAM microscopy's ability to distinguish vascular endothelial cells, pericytes, and astrocytes in a human blood-brain barrier microfluidic model, enabling further analysis and characterization of multicellular interactions, metabolic states, and depth-dependent changes.
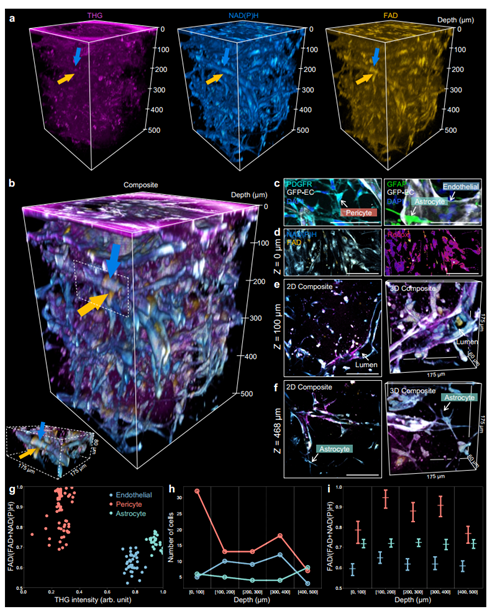
Figure 5. dSLAM for deep metabolic and structural imaging [1]
To test dSLAM's capacity for capturing faster dynamics, this study tracked monocyte behavior in vitro, simulating immune cell recruitment in living vascular networks. As shown in Figure 6, time-lapse imaging at 0.5 MHz pixel rate, 2 Hz frame rate, and 0.12 × 0.12 mm field of view enabled simultaneous monitoring of multicellular metabolic and motile behaviors.
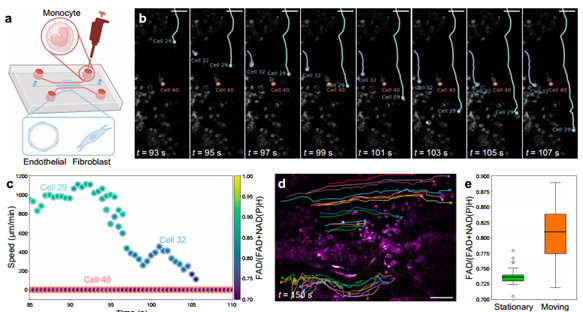
Figure 6. dSLAM for dynamic metabolic and structural imaging [1]
This study utilizes MMF-based wavelength conversion to generate high-peak-power pulses in the 1100 ± 25 nm band, extending the practical imaging depth of NAD(P)H in living engineered human multicellular microtissues to 700 µm. This increased depth enables observation of thicker three-dimensional tissue structures and metabolic processes and structural features of small model organisms. Furthermore, the compact slide-in fiber shaper MMF flexibly delivers high-peak-power pulses with near-Gaussian beam profiles, offering potential for miniaturization and widespread applications compared to previous methods.
References:
[1] Liu, K., Cao, H., Shashaty, K., Yu, L. Y., Spitz, S., Pramotton, F. M., ... & You, S. (2024). Deep and dynamic metabolic and structural imaging in living tissues. Science Advances, 10(50), eadp2438.
[2] Qiu, T., Cao, H., Liu, K., Yu, L. Y., Levy, M., Lendaro, E., ... & You, S. (2024). Spectral-temporal-spatial customization via modulating multimodal nonlinear pulse propagation. Nature Communications, 15(1), 2031.