Multiphoton microscopy imaging technology XLVI: Powerful SHG biomedical imaging technology
![]() 12/04 2024
12/04 2024
![]() 607
607
Over the past two decades, Second Harmonic Generation (SHG) microscopy has emerged as a pivotal technique in optical imaging, finding numerous applications in materials and biomedical sciences [1]. SHG is based on a second-order nonlinear optical process that generates signals only in materials with non-centrosymmetric structures. This unique imaging requirement endows SHG microscopy with high specificity. Collagen, one of the most prevalent proteins in human tissues, comprises three α-chains at the molecular level (Figure 1). In certain collagen types (primarily Types I and II), these triple helices spontaneously self-assemble into highly organized collagen fibers, producing robust SHG signals, whereas non-fibrous collagens (like Type IV) are not visible under SHG microscopy.
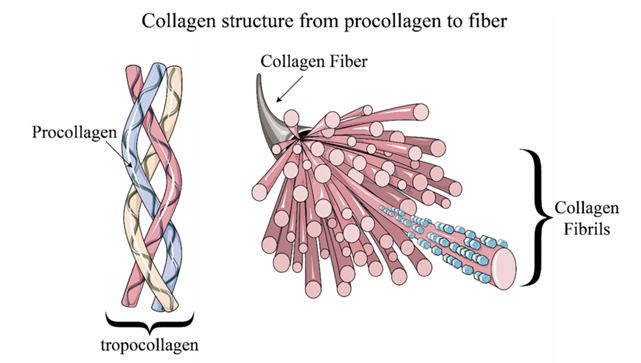
Figure 1: Hierarchical structure of collagen [1] The coherence of the SHG process imbues SHG images with rich biological sample information. Below is a brief overview of advanced SHG microscopy techniques [1].
Forward over backward second harmonic generation (F/B-SHG) [1] SHG signals are generally categorized into forward F-SHG and backward B-SHG signals. Since SHG signal generation is a coherent process, phase information is crucial. Phase-matching conditions are often challenging to meet in biological samples. For F-SHG signals, the coherence length (L_C) is relatively large (several micrometers), effectively revealing ordered structures at the SHG wavelength scale. In contrast, the coherence length (L_C) for B-SHG signals is much smaller (tens of nanometers), making pure B-SHG signals typically very weak but capable of revealing smaller structures. F/B-SHG microscopy leverages the directional advantages of SHG radiation patterns. Figures 2(a-d) illustrate that as dipoles (green arrows) stack within the focal volume, F-SHG intensity increases compared to B-SHG. Figures 2(e, f) show forward and backward SHG images of tendon tissue, respectively, while Figures 2(g, h) depict the changes in forward and backward SHG signals along the longitudinal and transverse directions, respectively.
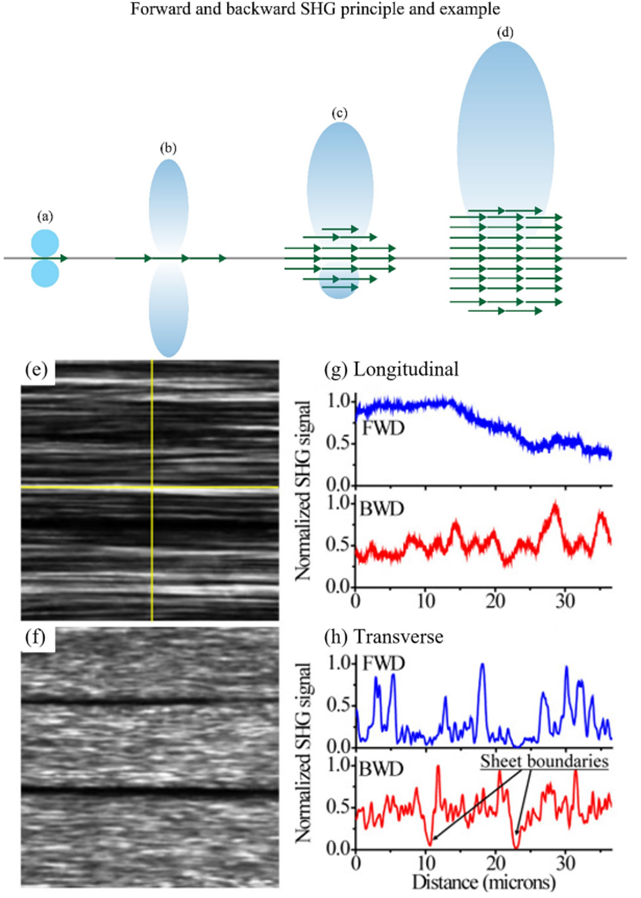
Figure 2: Principle and result examples of F/B SHG imaging [1]
Polarization-resolved second harmonic generation (P-SHG) [1] Collagen fibers are typically cylindrical. When linearly polarized laser light with a polarization angle μ relative to the x-axis propagates along the z-axis and the collagen fiber has an azimuth angle θ relative to the x-axis (see Figure 3(a) for details), the SHG signal intensity at each pixel in the image is given by: Equation (1) shows that varying the polarization angle of the incident light significantly affects the SHG signal intensity, which contains information about the orientation of biomacromolecules. By combining the advantages of SHG microscopy (high specificity and contrast) with polarization measurement (sensitivity to molecular alignment), P-SHG can be applied to collagen, providing a more accurate revelation of the complex hierarchical structure of fibers in the image plane. Figure 3(a) shows a schematic of a typical P-SHG microscope setup, where a quarter-wave plate and a half-wave plate optimize the linear polarization of the laser and change its polarization angle. Figure 3(b) presents an example of quantifying in-plane collagen fiber orientation using P-SHG microscopy.
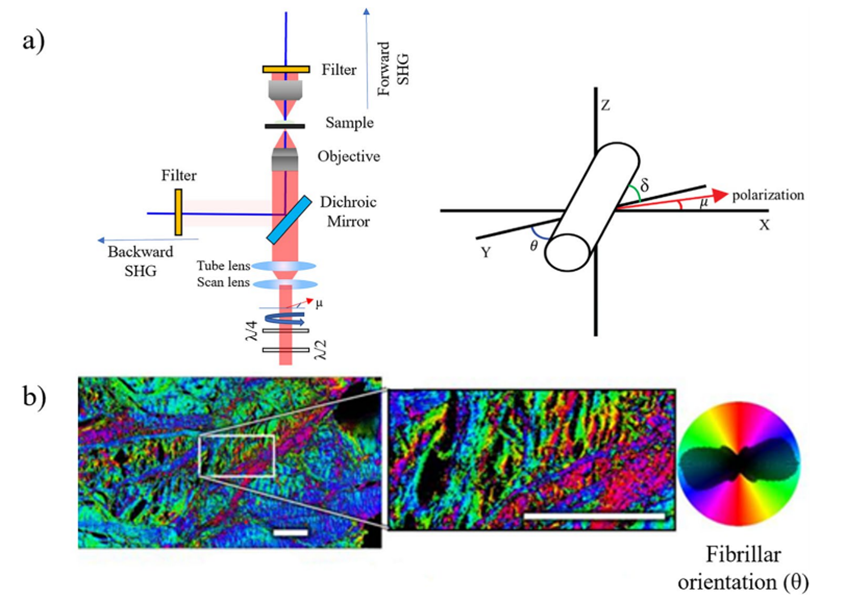
Figure 3: (a) Schematic of a typical P-SHG microscope setup, (b) Example of collagen fiber orientation measurement in an adult horse specimen using P-SHG microscopyCircular dichroism second harmonic generation (CD-SHG) [1] In addition to P-SHG, using left- and right-handed circularly polarized lasers can extract circular dichroism SHG, whose signal intensity is defined as: Similar to circular dichroism detected by linear microscopy, CD-SHG also requires non-zero optical activity (related to chiral symmetry). CD-SHG can be used to detect the three-dimensional polarity of collagen, as shown in Figure 4, which images collagen in a transverse section of a human cornea.
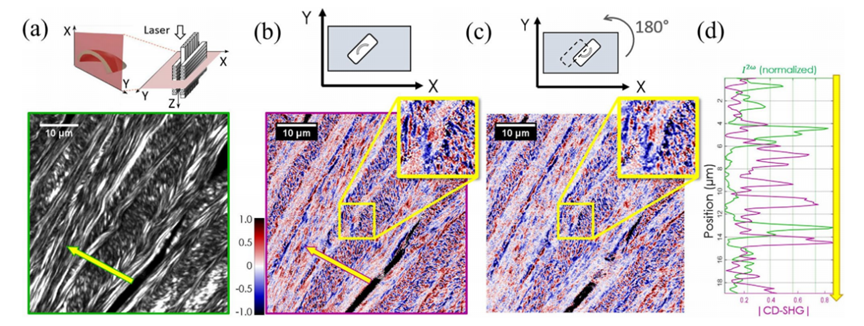
Figure 4: Example of human corneal transverse imaging using CD-SHGStokes vector-based second harmonic generation microscopy [1] While SHG microscopy can characterize sample responses to different linearly polarized lights and CD-SHG microscopy can characterize responses to different circularly polarized lights, these methods do not provide a complete response to all polarizations (e.g., incoherent, partially polarized, and unpolarized light). The Stokes-Mueller matrix formalism offers a more comprehensive description of a material's polarization response. The polarization state of light can be fully described by a 4×1 Stokes vector S, where different subscripts represent SHG signal intensities under incident laser polarizations at 0°, 90°, 45°, -45°, and left/right circular polarizations. The four elements of the matrix are normalized to lie within the range of -1 to 1. Important polarization parameters such as the degree of polarization (DOP), degree of linear polarization (DOLP), and degree of circular polarization (DOCP) can be derived from the vector S. Figure 5 shows imaging results of collagen fibers using this method, revealing more comprehensive orientation information. One of the main drawbacks of Stokes vector-based SHG microscopy is its limitation to forward detection configurations, necessitating thinner samples. Additionally, this method assumes a linear relationship between the incident laser and SHG signal but still fails to provide a complete polarization response of the sample.
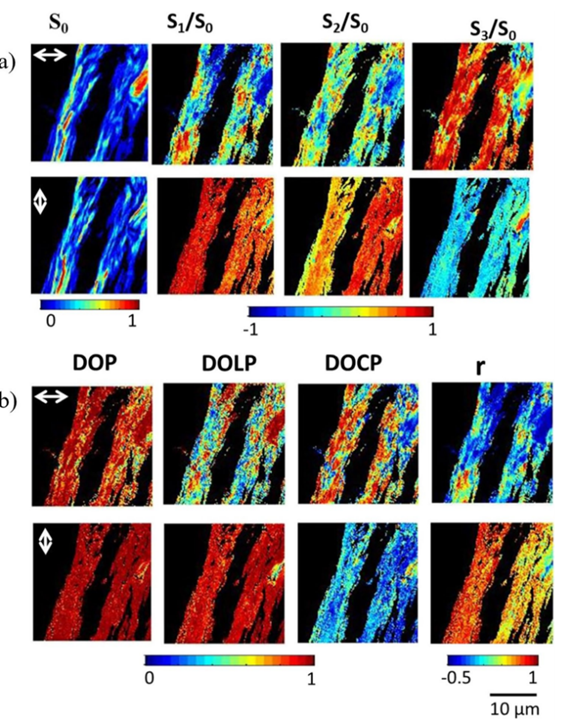
Figure 5: Imaging of collagen fibers using Stokes vector-based SHG microscopy [1]Interferometric second harmonic generation (I-SHG) [1] While the coherence of SHG is advantageous for providing additional sample information, it can also be a weakness as patterns seen on SHG images result from complex interference, potentially causing severe imaging artifacts and obscuring the actual underlying structure of biological samples (as seen in the black regions in Figures 2(e) and 2(f)). Therefore, to extract quantitative information, it is necessary to measure the local polarity within the sample. Observing Equation (4), a phase reversal leads to a π phase shift in the emitted SHG signal, indicating that the signal's phase preserves the polarity characteristics within the sample, which can be mapped to each pixel in the image. I-SHG detects the relative polarity of harmonic molecules by directly measuring the phase. This method relies on the combination of two SHG signals: one from a reference nonlinear crystal placed in front of the microscope and the other from the sample, which interfere with each other (see Figure 6(a)). Since both SHG beams are spatially and temporally coherent, the total intensity on the detector follows the usual two-wave interference equation:
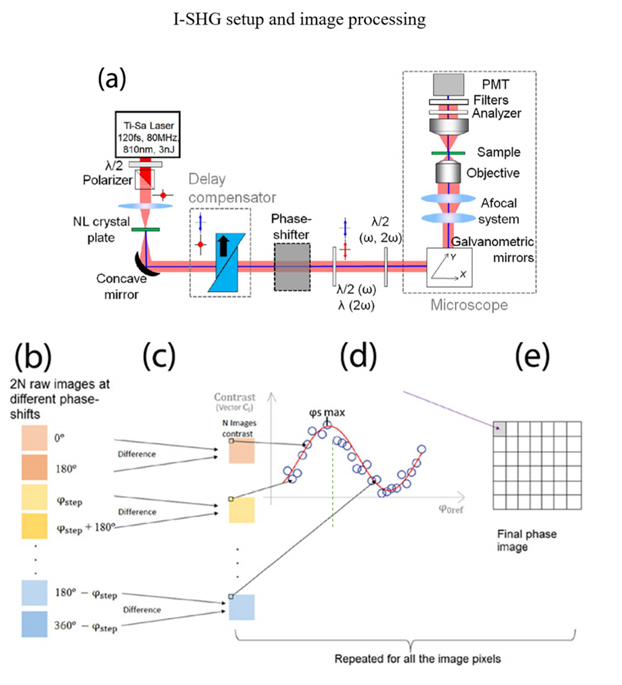
Figure 6: I-SHG setup and imaging procedure [1] By adjusting the phase difference between the two beams, interferograms can be recorded, and the cosine argument (i.e., relative phase) and its product factor (interference contrast) can be extracted by fitting the experimental curve (Figure 6(d)).Wide-field SHG imaging [1] Scanning SHG imaging, a well-established method successfully applied in numerous applications over the years, has limitations due to the low photon count per frame per second. This drawback hinders its application in label-free imaging of very fast biological processes (on the millisecond timescale). To overcome this limitation, wide-field SHG imaging emerged. As shown in Figure 7, the sample is placed slightly above the focal point, enabling a larger field of view. At this point, the entire region of interest is illuminated simultaneously, enabling parallel photon emission.
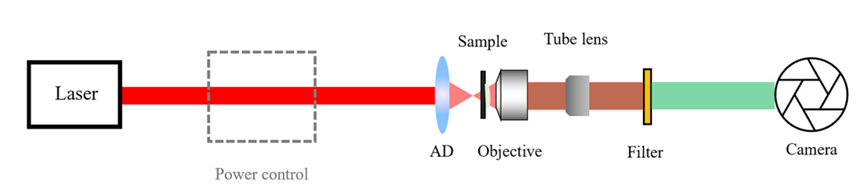
Figure 7: Typical wide-field microscope setup [1] This review introduces various advanced SHG imaging techniques that harness the advantages of SHG coherence, tomography, and high image contrast, shining in the study of biological tissues such as collagen, microtubules, and nerve cells. SHG and nonlinear optical microscopy imaging modalities provide a wealth of information that traditional linear or incoherent optical imaging techniques cannot easily obtain. With advancements in optics, machine learning, and laser technology, nonlinear imaging modalities will become better and simpler over time, opening new horizons for fundamental science and biomedical applications.
References: [1] Aghigh, A., Bancelin, S., Rivard, M. et al. Second harmonic generation microscopy: a powerful tool for bio-imaging. Biophys Rev 15, 43–70 (2023).