Multiphoton Microscopy Imaging Technique 51: Fast Synchronous Multimodal Optical Microscopy
![]() 11/10 2025
11/10 2025
![]() 600
600
The human brain is composed of tens of billions of neurons. These neurons communicate via electrical and chemical signals, laying the physiological foundation for complex functions such as thinking, memory, emotion, and behavior. For a considerable period, however, the scientific community has been in need of a tool that can simultaneously observe neuronal structure, metabolism, and biochemical changes with high resolution under non-interfering conditions. While traditional fluorescent labeling and gene-editing techniques are potent, they frequently disrupt the original physiological state of cells and find it challenging to capture multiple physiological processes at the same time. Consequently, the development of a label-free, multimodal, high-speed imaging technique has emerged as a significant challenge in the fields of neuroscience and biomedical imaging.
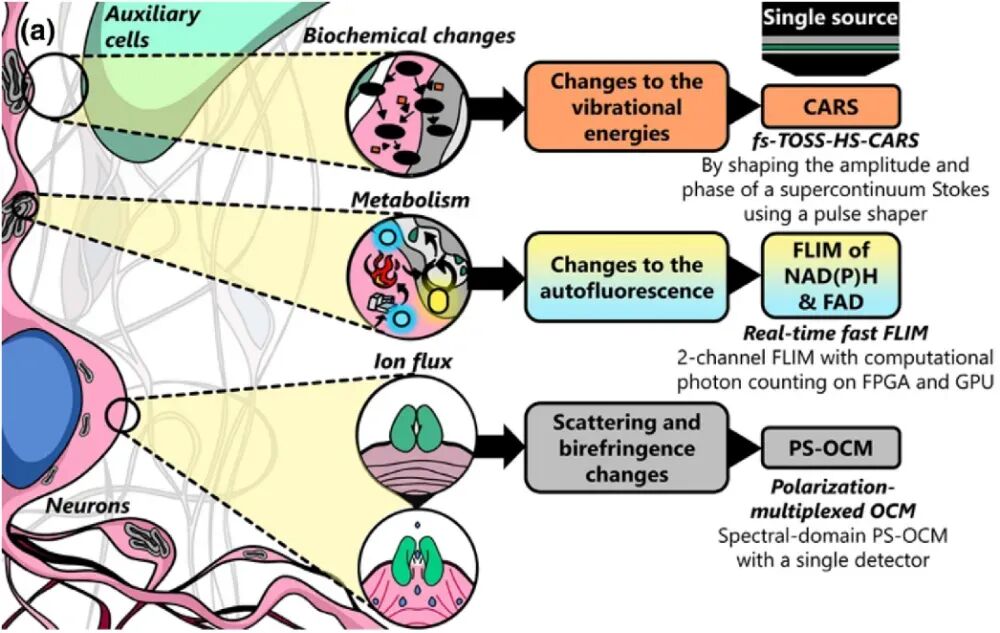
Figure 1. Various physical, metabolic, and biochemical changes occurring in the neuronal microenvironment [1]. The team led by Stephen Boppart at the University of Illinois at Urbana-Champaign has put forward a novel multimodal optical microscopy system named VAMPIRE, an acronym for “Versatile Autofluorescence lifetime, Multiharmonic generation, Polarization-sensitive Interferometry, and Raman imaging in epi-detection”. This system is capable of simultaneously acquiring four distinct physical and chemical signals: Scattered light: It detects changes in the cellular refractive index, which are correlated with electrical activity. Birefringence: It characterizes orientation changes in cell membranes or fibrous structures. Autofluorescence: It originates from metabolic cofactors NAD(P)H and FAD, enabling the assessment of cellular energy status. Raman scattering: It provides molecular vibration information, facilitating the analysis of the local biochemical environment. All these signals are excited by the same laser source. Through precise optical path design and computational imaging techniques, synchronous, high-speed, and high-resolution imaging is achieved.
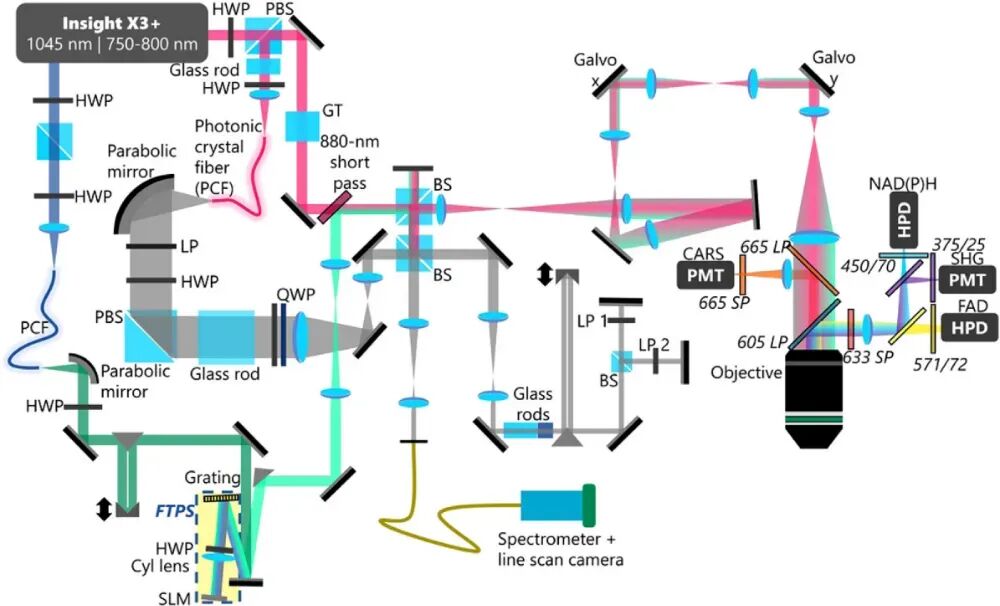
Figure 2. Schematic diagram of the VAMPIRE microscope system [1]. To achieve the simultaneous acquisition of the aforementioned multiple signals, the VAMPIRE system integrates various imaging modalities, including Optical Coherence Microscopy (OCM), Multiharmonic Imaging, Polarization-Sensitive Interferometry, Fluorescence Lifetime Imaging Microscopy (FLIM), and Coherent Anti-Stokes Raman Scattering (CARS) Imaging. The system employs a titanium-sapphire laser as the excitation source and combines it with a photonic crystal fiber to generate a supercontinuum spectrum. A Fourier transform pulse shaper is utilized to control the Stokes light in both the frequency and time domains, enabling the synchronous acquisition and processing of multimodal signals. Figure 2 illustrates the system structure of the VAMPIRE microscope. The gray optical path represents the Optical Coherence Microscopy module. Based on low-coherence interference principles, this module reconstructs the microscopic structure of samples by detecting interference signals between scattered light returned from different depths of the sample. To achieve high-resolution imaging, the system requires a light source with a relatively broad bandwidth. In this system, a titanium-sapphire laser with a central wavelength of 770 nm and a repetition rate of 80 MHz serves as the excitation source. Part of the output laser is coupled into a photonic crystal fiber to generate a supercontinuum spectrum with a bandwidth of approximately 200 nm. Subsequently, a series of optical components, including polarization beam splitters and dichroic mirrors, split and combine the beam, ultimately directing it into a linear scanning device. In the CARS imaging modality, the green optical path represents the Stokes light, while the pink optical path represents the pump light. Injecting a 1.3 W, 1045 nm laser into the photonic crystal fiber generates a supercontinuum spectrum spanning 200 nm (base width). The system employs a custom-designed Fourier transform pulse shaper, which consists of a diffraction grating and a spatial light modulator. This shaper independently controls the amplitude and phase of the Stokes light at different wavelengths, ensuring spectral resolution while maintaining femtosecond pulse width. This technique is known as TOSS-CARS [2]. After shaping, the Stokes light is tuned to cover several specific vibrational frequencies (e.g., 2830 cm⁻¹, 2930 cm⁻¹), interacting with the pump light to achieve specific imaging of different biochemical components, such as the CH stretching vibration region. Additionally, another 770 nm femtosecond laser in the system excites the autofluorescence of FAD and NAD(P)H and generates second harmonic signals. Signals from various imaging modalities are collected through a precisely configured filter system, which effectively separates signals from different imaging channels and minimizes spectral overlap, demonstrating the rationality of the system's optical path design.
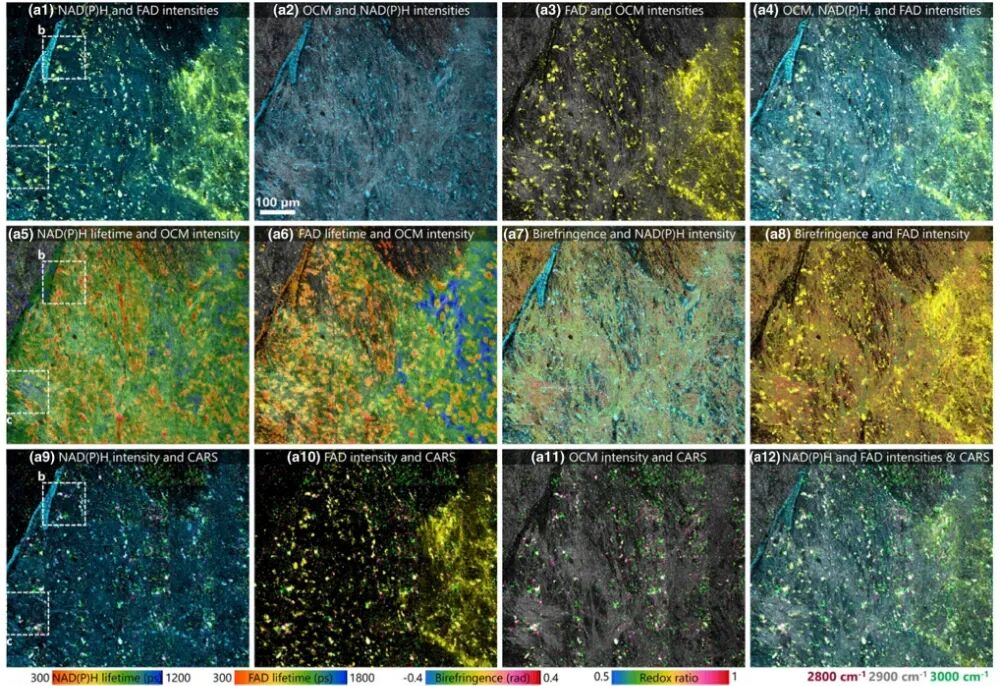
Figure 3. Images of slices near the mouse cerebral cortex captured using the VAMPIRE microscope [1]. Researchers employed the VAMPIRE system to image slices of the mouse cerebral cortex and retina (Figure 3), demonstrating its powerful capability in analyzing the structural, metabolic, and biochemical properties of the neuronal microenvironment. For instance, after adding glutamate stimulation to brain slices, the system successfully captured the dynamic responses of different neuronal clusters and identified five regions with distinct metabolic response patterns through clustering analysis. These patterns were further correlated with the lipid/protein ratios revealed by CARS, highlighting the advantages of multimodal imaging in associating structure with function.
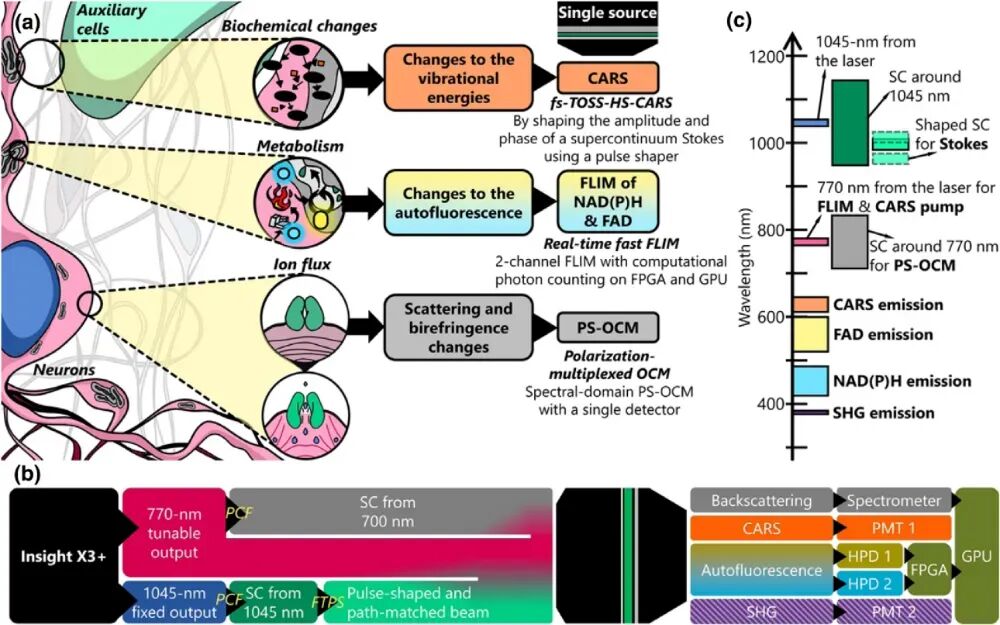
Figure 4. Simplified schematic diagram of single-source simultaneous detection in the VAMPIRE microscope [1]. As a novel label-free, high-speed, synchronous multimodal optical imaging platform, the VAMPIRE microscope provides unprecedented multidimensional information for analyzing the neuronal microenvironment. It aids in a deeper understanding of neural activity and the underlying metabolic mechanisms, marking a crucial step toward high-throughput optical physiology. In the future, it is expected to find wide applications in neuroscience, cancer biology, preclinical research, and other fields.
References:
[1] Iyer R R, Sorrells E J, Yang L, et al. Exploring the structure, metabolism, and biochemistry of the neuronal microenvironment label-free using fast simultaneous multimodal optical microscopy. Optica, 2024, 11(9): 1352-1367. DOI: 10.1364/OPTICA.532367.
[2] Yang L, Iyer R R, Sorrells E J, et al. Temporally optimized and spectrally shaped hyperspectral coherent anti-Stokes Raman scattering microscopy. Optics Express, 2024, 32(7): 11474-11490. DOI: 10.1364/OE.517417.






